Core Sup | Premium Health & Wellness Supplements
Mounjaro KwikPen Tirzepatide 15mg/0.6ml, Four-Dose Pre-Filled Injection Pen For Subcutaneous Injection
Mounjaro KwikPen Tirzepatide 15mg/0.6ml, Four-Dose Pre-Filled Injection Pen For Subcutaneous Injection
❇️ Product Specifications (Read More)
❇️ Product Specifications (Read More)
Mounjaro® (tirzepatide injection) is a once-weekly prescription medicine designed to improve blood sugar control in adults with type 2 diabetes. It is used alongside a healthy diet and regular exercise and represents a new generation of treatment that targets two important hormone pathways to help regulate glucose and support weight management.
How Mounjaro® Works
Mounjaro® is a dual GIP and GLP-1 receptor agonist. This means it activates two incretin hormones:
-
GIP (glucose-dependent insulinotropic polypeptide)
-
GLP-1 (glucagon-like peptide-1)
By acting on both, Mounjaro® helps the body:
-
Release more insulin when blood sugar is high
-
Reduce the amount of glucagon, a hormone that raises blood sugar
-
Slow down stomach emptying, which may reduce appetite
This dual action provides powerful effects on blood sugar control and weight reduction.
Dosage Forms and Strengths
Mounjaro® is supplied as a clear, colorless to slightly yellow solution for subcutaneous injection.
-
Single-Dose Prefilled Pen or Vial:
-
2.5 mg / 0.5 mL
-
5 mg / 0.5 mL
-
7.5 mg / 0.5 mL
-
10 mg / 0.5 mL
-
12.5 mg / 0.5 mL
-
15 mg / 0.5 mL
-
-
Mounjaro® KwikPen® (Multi-Dose Prefilled Pen):
-
Contains 4 fixed doses per pen
-
Available in 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg strengths (each in 0.6 mL solution)
-
Recommended Dosing
-
Starting Dose: 2.5 mg once weekly for 4 weeks
-
Step-Up Schedule: Increase to 5 mg once weekly; if needed, increase in 2.5 mg increments after at least 4 weeks on the current dose
-
Maximum Dose: 15 mg once weekly
Administration Guidelines:
-
Inject subcutaneously into abdomen, thigh, or upper arm
-
Rotate injection sites weekly
-
Can be given with or without meals at any time of the day
-
Do not administer daily, intramuscularly, or intravenously
Safety Information & Warnings
-
Thyroid C-Cell Tumors
Tirzepatide has caused thyroid tumors in animal studies. The risk in humans is unknown.-
Do not use if you or a family member has a history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
-
-
Pancreatitis
Cases of acute pancreatitis have been reported. Discontinue immediately if severe abdominal pain occurs. -
Hypoglycemia
Low blood sugar may occur when used with insulin or sulfonylureas. Dose adjustments may be required. -
Gastrointestinal Side Effects
Nausea, vomiting, diarrhea, and constipation are common. Severe cases may cause dehydration and kidney complications. -
Pregnancy and Breastfeeding
Contraindicated during pregnancy and breastfeeding. Women of childbearing age should use effective contraception during treatment and for at least 1 month after the last dose. Oral contraceptives may be less effective, so use a barrier or non-oral method during dose escalation.
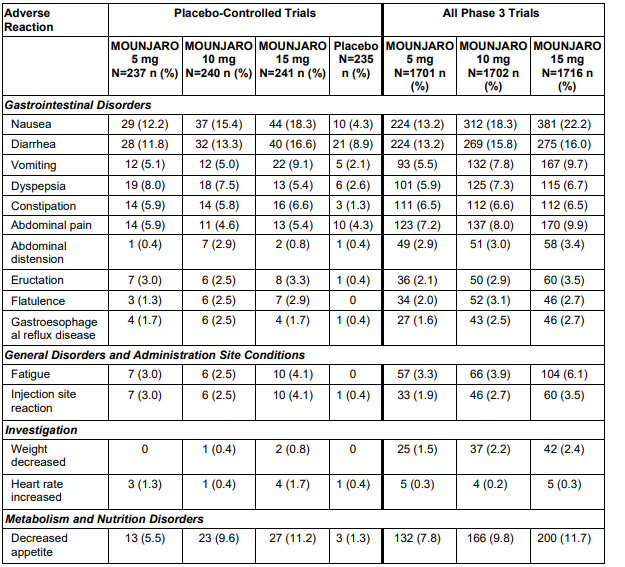
Common Side Effects
The most frequently reported side effects are gastrointestinal and are usually mild to moderate:
-
Nausea
-
Diarrhea
-
Vomiting
-
Decreased appetite
-
Constipation
-
Indigestion (dyspepsia)
-
Abdominal pain
Storage Instructions
-
Store in a refrigerator (2°C – 8°C / 36°F – 46°F)
-
Do not freeze
-
Once opened, a pen may be kept at room temperature (up to 30°C) for up to 30 days
No se pudo cargar la disponibilidad de retiro
 Money Back *up to 30 days
Money Back *up to 30 days
 Premium Quality
Premium Quality
 Worldwide Fast Shipping
Worldwide Fast Shipping
Share
Craziest fat burner i have ever used
















