Core Sup | Premium Health & Wellness Supplements
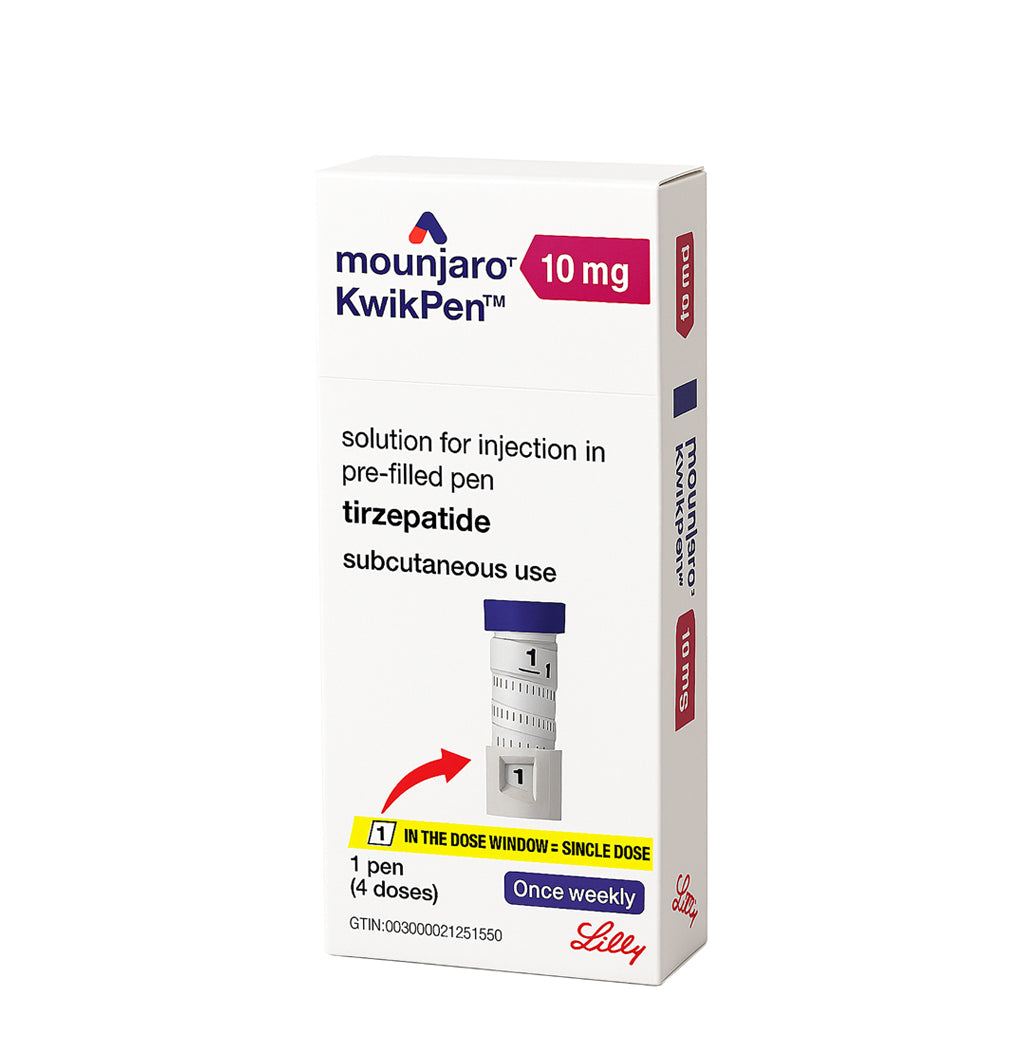
Mounjaro KwikPen Tirzepatide 10 mg/0.6ml, Four-Dose Pre-Filled Injection Pen For Subcutaneous Injection
Mounjaro KwikPen Tirzepatide 10 mg/0.6ml, Four-Dose Pre-Filled Injection Pen For Subcutaneous Injection
❇️ Product Specifications (Read More)
❇️ Product Specifications (Read More)
Mounjaro® (tirzepatide injection)
is a four-dose pre-filled injection pen for subcutaneous administration, designed to improve blood sugar control in adults with type 2 diabetes. As a dual GIP and GLP-1 receptor agonist, it enhances insulin release when glucose levels are high, reduces glucagon secretion, and slows gastric emptying. Used alongside diet and exercise, it supports effective glucose regulation and weight management, offering an advanced approach to diabetes care.
How Mounjaro® Works
Mounjaro® is a dual GIP and GLP-1 receptor agonist. This means it activates two incretin hormones:
-
GIP (glucose-dependent insulinotropic polypeptide)
-
GLP-1 (glucagon-like peptide-1)
By acting on both, Mounjaro® helps the body:
-
Release more insulin when blood sugar is high
-
Reduce the amount of glucagon, a hormone that raises blood sugar
-
Slow down stomach emptying, which may reduce appetite
This dual action provides powerful effects on blood sugar control and weight reduction.
Dosage Forms and Strengths
Mounjaro® is supplied as a clear, colorless to slightly yellow solution for subcutaneous injection.
-
Single-Dose Prefilled Pen or Vial:
-
2.5 mg / 0.5 mL
-
5 mg / 0.5 mL
-
7.5 mg / 0.5 mL
-
10 mg / 0.5 mL
-
12.5 mg / 0.5 mL
-
15 mg / 0.5 mL
-
-
Mounjaro® KwikPen® (Multi-Dose Prefilled Pen):
-
Contains 4 fixed doses per pen
-
Available in 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg strengths (each in 0.6 mL solution)
-
Recommended Dosing
-
Starting Dose: 2.5 mg once weekly for 4 weeks
-
Step-Up Schedule: Increase to 5 mg once weekly; if needed, increase in 2.5 mg increments after at least 4 weeks on the current dose
-
Maximum Dose: 15 mg once weekly
Administration Guidelines:
-
Inject subcutaneously into abdomen, thigh, or upper arm
-
Rotate injection sites weekly
-
Can be given with or without meals at any time of the day
-
Do not administer daily, intramuscularly, or intravenously
Safety Information & Warnings
-
Thyroid C-Cell Tumors
Tirzepatide has caused thyroid tumors in animal studies. The risk in humans is unknown.-
Do not use if you or a family member has a history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
-
-
Pancreatitis
Cases of acute pancreatitis have been reported. Discontinue immediately if severe abdominal pain occurs. -
Hypoglycemia
Low blood sugar may occur when used with insulin or sulfonylureas. Dose adjustments may be required. -
Gastrointestinal Side Effects
Nausea, vomiting, diarrhea, and constipation are common. Severe cases may cause dehydration and kidney complications. -
Pregnancy and Breastfeeding
Contraindicated during pregnancy and breastfeeding. Women of childbearing age should use effective contraception during treatment and for at least 1 month after the last dose. Oral contraceptives may be less effective, so use a barrier or non-oral method during dose escalation.
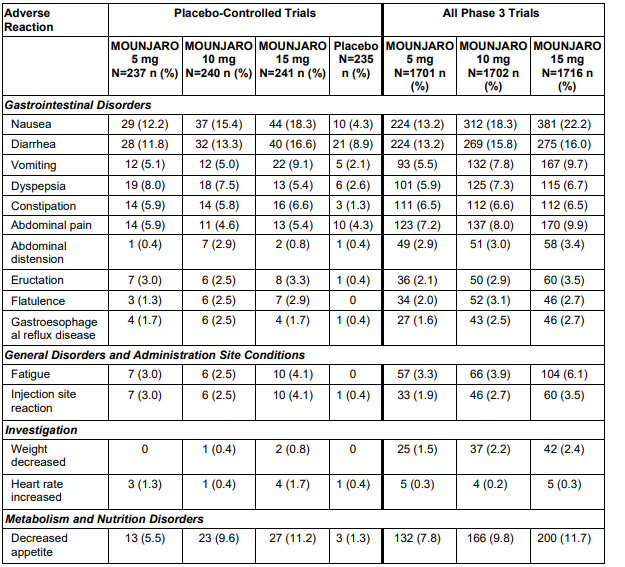
Common Side Effects
The most frequently reported side effects are gastrointestinal and are usually mild to moderate:
-
Nausea
-
Diarrhea
-
Vomiting
-
Decreased appetite
-
Constipation
-
Indigestion (dyspepsia)
-
Abdominal pain
Storage Instructions
-
Store in a refrigerator (2°C – 8°C / 36°F – 46°F)
-
Do not freeze
-
Once opened, a pen may be kept at room temperature (up to 30°C) for up to 30 days
Kan beschikbaarheid voor afhalen niet laden
 Money Back *up to 30 days
Money Back *up to 30 days
 Premium Quality
Premium Quality
 Worldwide Fast Shipping
Worldwide Fast Shipping
Share